Protocol for Preparing Adherent Cells such as 3T3 and HEK:¶
Prepare Media For Priming and Flushing Cell Channel of Chrono-Seq Device¶
- Put the DMEM with 1% PEN-STEP Only, DPBS, and TrypLE express bottles in the 37°C water bath in the Cell culture room. Remember we need NO Fetal Bovine Serum (FBS) in our media for these experiments.
- Turn on the UV for the Cell cutlure hood and wait 15 minutes before you start.
- You need about 5ml of media for each timepoint or injection. So for 16 Timepoints you will need about 80ml combined for the Flush containers. Increase this volume for experiments with more timepoints/injections.
- Inside the Cell Culture Hood. Filter between 100-150ml of DMEM with 1% PEN-STREP using 40μm Cell Strainers depending on the number of timepoints/injections for your experiment.
- Label and Transfer Filtered Liquid to 50ml Tubes for Chrono-Seq Device:
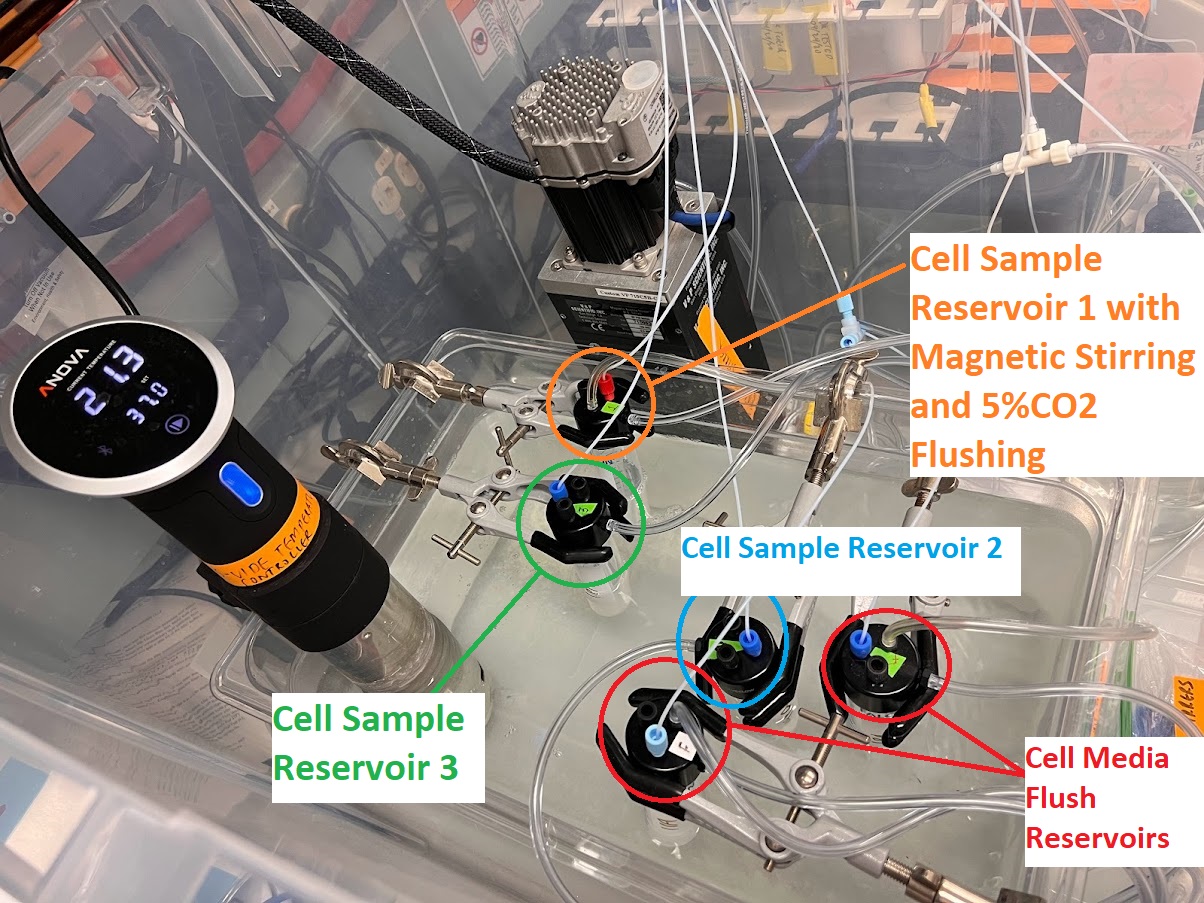
- Label two Sterile/Particle-Free 50ml Falcon Tubes: Cell Media Flush Reservoir: DMEM 1% PEN-STEP
- Transfer between 25ml-50ml each to the two 50ml Falcon Tubes depending on number of timepoints/injections for the Cell Media Flush Reservoirs.
- Label three separate Sterile/Particle-Free 50ml Falcon Tubes Cell Sample Reservoir 1: DMEM 1% PEN-STEP, Cell Sample Reservoir 2: DMEM 1% PEN-STEP, and Cell Sample Reservoir 3.: DMEM 1% PEN-STEP
- Transfer 15ml each to these three 50ml Falcon Tubes for the Cell Sample Reservoirs.
- Store these 5 Falcon Tubes in Tube racks on your Bench for loading into the Chrono-Seq Device.
✶ Note: You don't need to perfrom the three steps above if you are only running the Bulk RNA seq protocol.
Cell Suspension Preparation¶
- Examine your cells under the microscope for Contamination.
- Remove the Media in the Cell Culture Vessels for 3T3 and HEK
- Wash the Cells gently with 10ml of DPBS twice. This step is to get rid of all the FBS(Fetal Bovine Serum). When washing make sure you don't detach the cells by directly dispensing liquid over the cells.
- Add 5 ml of TrypLE express to both culture vessels. Make sure there is an even coat on the Cells.
- Place in the incubator for 5 minutes at 37°C.
- Remove the cells and check below the Culture Vessel to see if the Cells are detached completely.
- Swirl the Culture Vessels in Circles to detach all the Cells.
- If the Cell density is very high it might be difficult to break the cells into a single-cell suspension. Put the Cells back into the incubator for an additional 3 minutes of digestion in this case.
- Pipette the Cells up and down using a 5ml pipette to break any remaining chunks.
- Transfer the Cells to two 15ml Tubes labeled with the name of the Cell Line on each tube.
- Spin down at 500g for 5 minutues.
- Remove the TrypLE express without disturbing the Cell pellets.
- Using a 5ml Pipette, resuspend in 5ml of Serum-Free DMEM with 1% PEN-STEP Only. Use DMEM with 1% PEN-STREP for the remaining steps as well.
- Now add another 5ml each to the 15ml tubes.
- Label two 50ml Tubes with the name of the Cell lines.
- Filter the cells using a 40μm Cell strainer.
- Pipette up and down to make sure the cells have a uniform concentration. Using a P1000, make a 1/20 Dilution using Sterile Tips in a 1.5ml Eppendorf Tube. Do this by adding 50μl of Cell Suspension to 950μl of Media.
- Mix the 1/20 Dilution throughly using the P1000 by pipetting up and down at least 5 times. This is to make sure the Cells have a uniform concentration. Now you can count the cells.
- Add 20μl of the 1/20 Dilution using a P20 to a Fuchs-Rosenthal Hemocytometer. Count at least 5 squares of the Fuchs-Rosenthal Hemocytometer.
- We need a final concentration of 215 cells/μl for both 3T3 and HEK. Make the Calculation for getting this final concentration.
- Since each Square of the Hemocytometer is 0.2μl. Five squares should give you the Concentration in the 1/20 Dilution per μl. Multiply this number by 20 to get the Approximate concentration in your Cell Stock in cells/μl.
- Make 50ml of 3T3 and 50ml of HEK @ 215 cells/μl. Calculate how much volume of cells is needed to make the this final dilution. Make sure the cells have a uniform concentration before making this final dilution.
- Remember to Label the Tubes with the Name of the Cell Line, Date, Media, Concentration of Cells, and Volume.
✶ OPTIONAL NOTE: You can make the Same Calculation for a Different Cell Concentration and Volumes if you wish.
For Species Mixing Experiment Using ChronoSeq Device:¶
- Make 50ml of a 50:50 Mix of 3T3 and HEK293 Cells by mixing 25ml of 3T3 and 25ml of HEK293 made in the previous step.
- Add a Clean Magnetic Stirring Disk to another 50ml Falcon Tube and add 35ml of 50:50 Mix to it. Label this Tube as : 3T3:HEK 50:50 Mix, Cell Reservoir 1, 35ml Total Volume
| Location of Magnetic Stirrers | Pouch with Magnetic Stirrers |
|---|---|
 |
 |
- You are now ready to run the Machine.