Protocol for Preparing Adherent Cells such as 3T3 and HEK for Production Experiments:¶
- Recipe for DMEM-BSA:
- 1X DMEM
- 1%PEN-STEP
- 0.02% BSA
The purpose of DMEM-BSA is to prevent the Cells from sticking to each other. Using a higher concentration of BSA might interfere with droplet breakage.
Prepare Media For Priming and Flushing Cell Channel of Chrono-Seq Device¶
- Put the DMEM with 1% PEN-STEP Only, DMEM with 1% PEN-STEP and 10%Fetal Bovine Serum(FBS) DPBS, and TrypLE express bottles in the 37°C water bath in the Cell culture room.
- Thaw one Aliquot of 5%BSA in DPBS on your bench. Protect the BSA from UltraViolet(UV) Light.
- Turn on the UV for the Cell cutlure hood and wait 15 minutes before you start.
- You need about 5ml of media for each timepoint or injection. So for 16 Timepoints you will need about 80ml combined for the Flush containers. Increase this volume for experiments with more timepoints/injections.
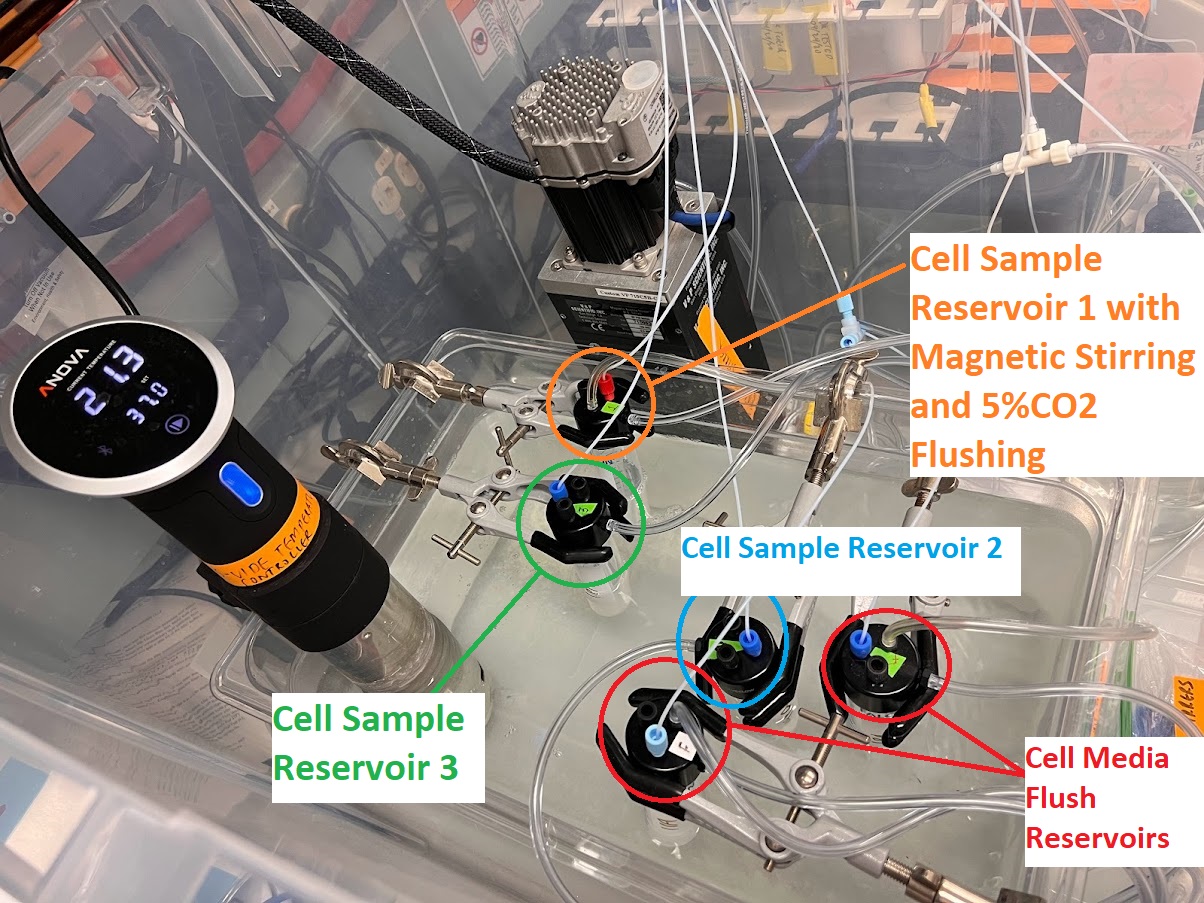
- Inside the Cell Culture Hood. Prepare between 100-150ml of DMEM-BSA depending on the number of timepoints/injections for your experiment.
- Using 5% BSA and DMEM with 1%PEN-STEP make fresh DMEM-BSA (0.02% BSA).
- Filter this DMEM-BSA with a 40μm Cell Strainer
Always make DMEM-BSA Fresh before the Experiment. Do not make Stocks in Advance!
- Label and Transfer Filtered Liquid to 50ml Tubes for Chrono-Seq Device:
- Label two Sterile/Particle-Free 50ml Falcon Tubes: Cell Media Flush Reservoir: DMEM-BSA
- Transfer between 25ml-50ml each to the two 50ml Falcon Tubes depending on number of timepoints/injections for the Cell Media Flush Reservoirs.
- Label three separate Sterile/Particle-Free 50ml Falcon Tubes Cell Sample Reservoir 1: DMEM-BSA, Cell Sample Reservoir 2: DMEM-BSA, and Cell Sample Reservoir 3.: DMEM-BSA
- Transfer 15ml each to these three 50ml Falcon Tubes for the Cell Sample Reservoirs.
- Store these 5 Falcon Tubes in Tube racks on your Bench for loading into the Chrono-Seq Device.
Cell Suspension Preparation and Regrowing Cell Surface Receptors¶
- Examine your cells under the microscope for Contamination.
- Remove the Media in the Cell Culture Vessels for 3T3 or HEK293.
- Wash the Cells gently with 10ml of DPBS two times.
- This step is to get rid of all the FBS(Fetal Bovine Serum).
- When washing make sure you don't detach the cells. Avoid detachment by not directly dispensing liquid over the cells.
- Add 5 ml of TrypLE express to both culture vessels. Make sure there is an even coat on the Cells.
- Place in the incubator for 5 minutes at 37°C.
- Remove the cells and check below the Culture Vessel to see if the Cells are detached completely.
- Swirl the Culture Vessels in Circles to detach all the Cells.
- If the Cell density is very high it might be difficult to break the cells into a single-cell suspension. Put the Cells back into the incubator for an additional 3 minutes of digestion in this case.
- Pipette the Cells up and down using a 5ml pipette to break any remaining chunks.
- Transfer the Cells to two 15ml Tubes labeled with the name of the Cell Line on each tube.
- Spin down at 500g for 5 minutues.
- Remove the TrypLE express without disturbing the Cell pellets.
- Using a 5ml Pipette, resuspend in 5ml of DMEM with 10%FBS and 1% PEN-STEP.
- Now add another 5ml each to the 15ml tubes.
- Label one 50ml Tube with the name of the Cell line.
- Filter the cells using a 40μm Cell strainer
| Location of Magnetic Stirrers | Pouch with Magnetic Stirrers |
|---|---|
 |
 |
- Add a Clean Magnetic Stirring Disk to another 50ml Falcon Tube and add 30ml of DMEM with 10%FBS and 1% PEN-STEP to it. Label this Tube as : HEK293 Suspension for Regrowth of Cell Surface Proteins
- Add the Filtered HEK293 Cell Suspension to this new tube and mix well with the pipette.
- Load this suspension into Cell Reservoir 1 and execute keep_suspension() schedule for 5 hours here.
Make a FBS-Free Cell Suspension after 5 hours¶
- Freshly Prepare enough Volume of DMEM-BSA for the remaining steps below. To prepare DMEM-BSA:
- Use 5% BSA and DMEM with 1%PEN-STEP make fresh DMEM-BSA (0.02% BSA).
- Filter this DMEM-BSA with a 40μm Cell Strainer
- Get the Cell Suspension from the Cell Reservoir 1. Replace the Tube with the DMEM-BSA that was loaded earlier.
- Inside the Cell Culture hood use a Magnet to Remove the magnetic stirring disk from the Cell Suspension.
TO DO Take a Picture or Video on How to Do This.
- Throughly Clean the Magnetic Stirring Disk with 70% Ethanol.
- Dry this Magnetic Stirring Disk using Particle Free Cleanroom Wipes or Paper Towels.
- Keep this Magnetic Stirring Disk inside the Cell Culture Hood on a Cleanroom wipe or Paper Towel.
- Spin down at 500g for 5 minutues.
- Wash the Cells Thrice using DMEM-BSA:
- Remove most of the media in the Falcon Tube without Disturbing the Cell Pellet.
- Resuspend the Cells in 25 ml of DMEM-BSA.
- Spin down at 500g for 5 minutes.
- Remove most of the media in the Falcon Tube without Disturbing the Cell Pellet.
- Resuspend the Cells in 25 ml of DMEM-BSA.
- Spin down at 500g for 5 minutes.
- Remove most of the media in the Falcon Tube without Disturbing the Cell Pellet.
- Resuspend the Cells in 10 ml of DMEM-BSA.
- Filter the cells using a 40μm Cell strainer.
- Pipette up and down to make sure the cells have a uniform concentration. Using a P1000, make a 1/20 Dilution using Sterile Tips in a 1.5ml Eppendorf Tube. Do this by adding 50μl of Cell Suspension to 950μl of DMEM-BSA.
- Mix the 1/20 Dilution throughly using the P1000 by pipetting up and down at least 5 times. This is to make sure the Cells have a uniform concentration. Now you can count the cells.
- Add 20μl of the 1/20 Dilution using a P20 to a Fuchs-Rosenthal Hemocytometer. Count at least 5 squares of the Fuchs-Rosenthal Hemocytometer.
- We need a final concentration of 600 cells/μl for Bulk or 215 cells/μl for Single-Cell. Make the Calculation for getting this final concentration.
- Since each Square of the Hemocytometer is 0.2μl. Five squares should give you the Concentration in the 1/20 Dilution per μl. Multiply this number by 20 to get the Approximate concentration in your Cell Stock in cells/μl.
- Make 40ml of HEK293 at 600cells/μl for Bulk Time-Series or 215 cells/μl for Single-Cell Experiments in DMEM-BSA. Calculate how much volume of cells is needed to make the this final dilution. Make sure the cells have a uniform concentration before making this final dilution.
- Remember to Label the Tube with the Name of the Cell Line, Date, Media:DMEM-BSA, Concentration of Cells, and Volume.
✶ OPTIONAL NOTE: You can make the Same Calculation for a Different Cell Concentration and Volumes if you wish.
- Add the Clean/Particle Free Magnetic Stirring Disk to another 50ml Falcon Tube and add 40ml of HEK293 suspension to it. Label this Tube as : HEK293 Suspension, Cell Reservoir 1, 40ml Total Volume
| Location of Magnetic Stirrers | Pouch with Magnetic Stirrers |
|---|---|
 |
 |
- You are now ready to run the Machine.