Protocol for suspending ChronoSeq/Dropseq Beads stored in Ethanol to 1X Lysis Buffer¶
Storage of ChronoSeq or Dropseq beads¶
- You should receive your beads suspended in Ethanol.
- Store these beads in a new box at -20°C before you start your experiments or for long term storage. Label the box with the Date you received the beads, the bead type and the batch number.
Prepare Lysis Buffer Stock¶
- We suggest preparing 3.33X Lysis Buffer Stock using this protocol, before you get started.
Best Practices:¶
- Remember to put back the reagents back to their storage location at 4°C or -20°C once you are done with using them.
- Get fresh new microtips/sterile-disposable pipettes after each step, unless mentioned otherwise.
- Important to avoid cross-contamination.
Equipment Used:¶
- Our lab uses the refrigerated Sorval ST8R Centrifuge. You can find CAD files to print some of the parts here.
- We bought the 50ml Inserts and Printed the Rest.
- You can modify the CAD files to print the 50ml Inserts as well.
- Use PLA or TPU for the prints.
- Microcentrifuge we used was the Fisherbrand™ accuSpin™ Micro 17/17R Microcentrifuge
- CELLTREAT Pipette Controller
- Labnet Vortex
- Eppendorf Pipettes 6-Pack
- Pipette Tips:
- Larger Volume Pipettes:
- Disposable Sterile Falcon Tubes:
- Disposable Sterile 250ml GL45 Bottles, Individually Wrapped
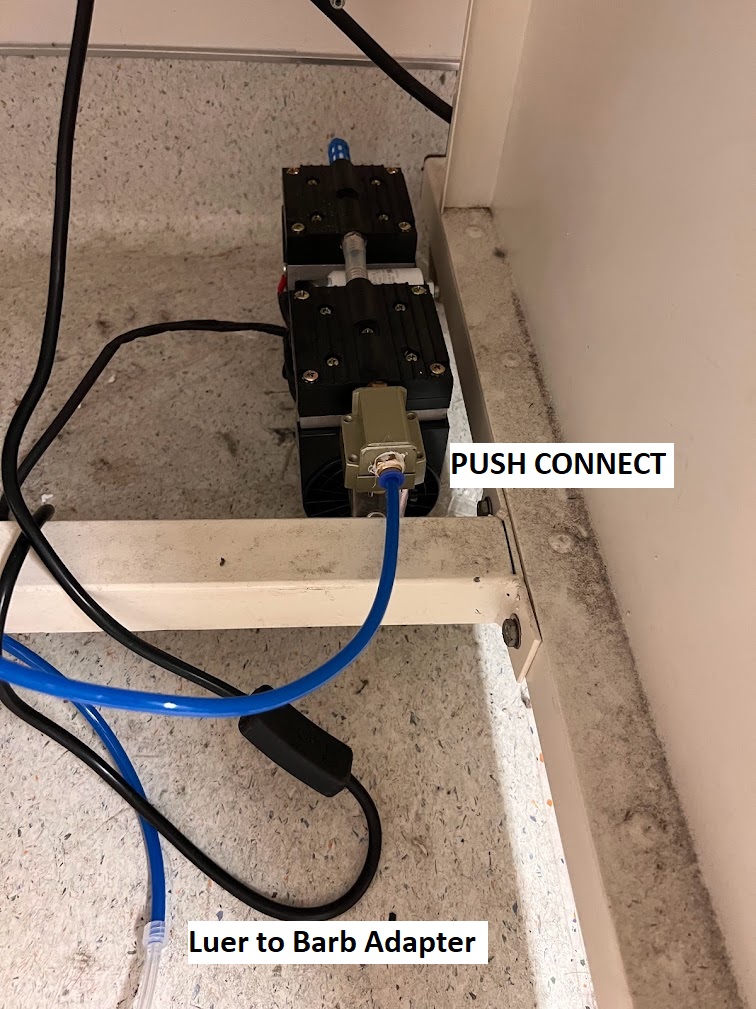
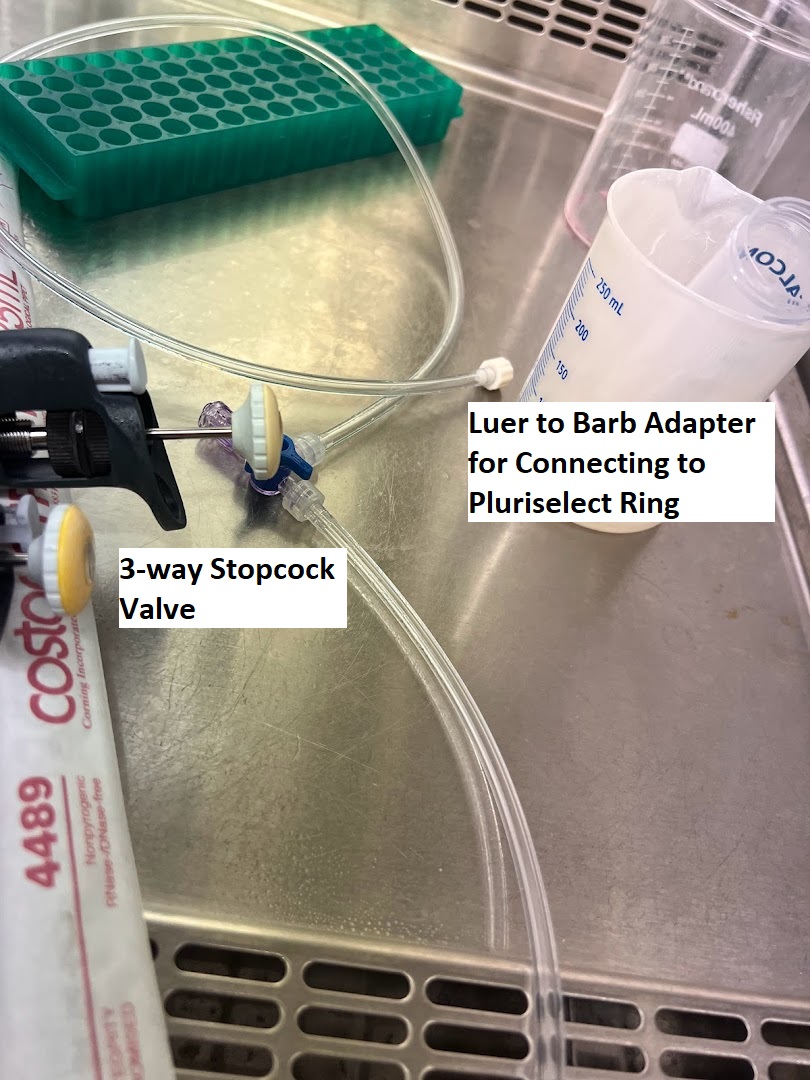
Vacuum System Setup and Assembly¶
- Assemble the Vacuum setup shown in the images below. You will need the following:
- 1 litre GL-45 Glass Bottle
- Pluriselect Connector Ring (Green Color)
- 3D Printed Cap for GL-45 Bottle
- 15μm Pluriselect Filter (Dark Gray Color)
- Vacuum Pump
- Push Connect fitting kit
- Luer Lock Barb Male Adapter
- Luer Lock Barb Female Adapter
- 3-Way Stop-Cock
- VICI 3 Port Bottle Cap
- 0.45μm Syringe Filter
- Delrin Plug 1/4-28 UNF
- Tygon Tubing 3/32" ID x 7/32" OD x 1/16" Wall
- 1/4-28 UNF to Barb Adapter
- Adjustable Wrench
Use Teflon Tape from Fittings Kit to get a better seal if necessary: